Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Tanaka, Kazuya; Yamaji, Keiko*; Masuya, Hayato*; Tomita, Jumpei; Ozawa, Mayumi*; Yamasaki, Shinya*; Tokunaga, Kohei; Fukuyama, Kenjin*; Ohara, Yoshiyuki*; Maamoun, I.*; et al.
Chemosphere, 355, p.141837_1 - 141837_11, 2024/05
In this study, biogenic Mn(IV) oxide was applied to remove Ra from mine water collected from a U mill tailings pond in the Ningyo-toge center. Just 7.6 mg of biogenic Mn(IV) oxide removed more than 98% of the  Ra from 3 L of mine water, corresponding to a distribution coefficient of 10
Ra from 3 L of mine water, corresponding to a distribution coefficient of 10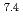 mL/g for Ra at pH 7. The obtained value was convincingly high for practical application of biogenic Mn(IV) oxide in water treatment.
mL/g for Ra at pH 7. The obtained value was convincingly high for practical application of biogenic Mn(IV) oxide in water treatment.
 -type layered manganese dioxide
-type layered manganese dioxideOkamoto, Norihiko*; Yoshisako, Hiroki*; Ichitsubo, Tetsu
Energy Storage Materials, 61, p.102912_1 - 102912_9, 2023/08
Times Cited Count:1 Percentile:52.07(Chemistry, Physical) through irreversible biogenic manganese oxide sequestration
through irreversible biogenic manganese oxide sequestrationTani, Yukinori*; Kakinuma, Satomi*; Chang, J.*; Tanaka, Kazuya; Miyata, Naoyuki*
Minerals (Internet), 11(1), p.53_1 - 53_14, 2021/01
Times Cited Count:4 Percentile:45.1(Geochemistry & Geophysics)In this study, we examined the Ba sequestration potential of enzymatically active biogenic Mn oxides (BMOs) with and without exogenous Mn
sequestration potential of enzymatically active biogenic Mn oxides (BMOs) with and without exogenous Mn . The BMOs readily oxidized exogenous Mn
. The BMOs readily oxidized exogenous Mn to produce another BMO phase, and subsequently sequestered Ba
to produce another BMO phase, and subsequently sequestered Ba at a pH of 7.0, with irreversible Ba
at a pH of 7.0, with irreversible Ba sequestration as the dominant pathway. Extended X-ray absorption fine structure spectroscopy and X-ray diffraction analyses demonstrated alteration from turbostratic to tightly stacked birnessite through possible Ba
sequestration as the dominant pathway. Extended X-ray absorption fine structure spectroscopy and X-ray diffraction analyses demonstrated alteration from turbostratic to tightly stacked birnessite through possible Ba incorporation into the interlayer.
incorporation into the interlayer.
Yu, Q.*; Tanaka, Kazuya; Kozai, Naofumi; Sakamoto, Fuminori; Tani, Yukinori*; Onuki, Toshihiko
ACS Earth and Space Chemistry (Internet), 2(8), p.797 - 810, 2018/08
Times Cited Count:14 Percentile:56.1(Chemistry, Multidisciplinary)Most of Mn oxides are biogenic and known to adsorb cesium (Cs) on the surface. This study investigated structural transformation of biogenic birnessite by accommodating commonly occurring natural heavy metals (Zn, Ni) during the formation of Mn oxides and the influence of those metals on the adsorption behavior of Cs on Mn oxides. It was found that the presence of heavy metals during bio-oxidation of Mn(II), followed by exposure to a low pH aqueous solution, increased the number of available layer vacancies, which consequently increased the adsorption capacity of Cs in the final product birnessite.
 thin films
thin filmsYanagida, Tsuyoshi*; Saito, Yuji; Takeda, Yukiharu; Fujimori, Atsushi*; Tanaka, Hidekazu*; Kawai, Tomoji*
Physical Review B, 79(13), p.132405_1 - 132405_4, 2009/04
Times Cited Count:11 Percentile:45.17(Materials Science, Multidisciplinary)Creating a bipolarity of semiconductors has been a key technology to develop recent advanced semiconductor devices. Although theoretical calculation predicts the presence of ferromagnetic electron-doped manganites with doping tetravalent cations, the ferromagnetic origin in experiments has been controversial due to the lack of direct experimental evidence. Here, we investigate the ferromagnetism in (La,Ce)MnO thin films by measuring the magnetic circular dichroism in soft X-ray absorption (XMCD). The XMCD measurements clarified that the ferromagnetism is not caused by the presence of Mn
thin films by measuring the magnetic circular dichroism in soft X-ray absorption (XMCD). The XMCD measurements clarified that the ferromagnetism is not caused by the presence of Mn but by self-hole doping for manganese.
but by self-hole doping for manganese.
Hotta, Takashi
Progress in Ferromagnetism Research, p.19 - 38, 2006/00
In manganites, the appearance of the ferromagnetic (FM) metallic phase has been usually rationalized by the so-called double-exchange mechanism, based on a strong Hund's rule coupling between mobile 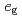 electrons and localized
electrons and localized 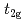 spins. Then, it has been believed for a long time that hole-doping is indispensable for explaining the FM metallic phase in manganites. However, it has been theoretically pointed out that a novel metal-insulator transition in the FM state can occur even for undoped manganites based on the
spins. Then, it has been believed for a long time that hole-doping is indispensable for explaining the FM metallic phase in manganites. However, it has been theoretically pointed out that a novel metal-insulator transition in the FM state can occur even for undoped manganites based on the 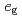 -orbital degenerate Hubbard model tightly coupled to Jahn-Teller distortions. In this article, the result is reviewed in detail.
-orbital degenerate Hubbard model tightly coupled to Jahn-Teller distortions. In this article, the result is reviewed in detail.
 Sr
Sr MnO
MnO studied by resonant inelastic X-ray scattering
studied by resonant inelastic X-ray scatteringIshii, Kenji; Inami, Toshiya; Owada, Kenji; Kuzushita, Kaori; Mizuki, Junichiro; Murakami, Yoichi; Ishihara, Sumio*; Endo, Yasuo*; Maekawa, Sadamichi*; Hirota, Kazuma*; et al.
Journal of Physics and Chemistry of Solids, 66(12), p.2157 - 2162, 2005/12
Times Cited Count:0 Percentile:0(Chemistry, Multidisciplinary)We have studied electronic excitations of hole-doped manganese oxides La Sr
Sr MnO
MnO (
(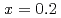 and
and 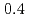 ) by resonant inelastic X-ray scattering at Mn K absorption edge. We observed in the excitation spectra that the Mott gap of La
) by resonant inelastic X-ray scattering at Mn K absorption edge. We observed in the excitation spectra that the Mott gap of La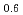 Sr
Sr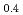 MnO
MnO was filled by hole-doping. In La
was filled by hole-doping. In La Sr
Sr MnO
MnO , which shows a metal-insulator transition, we found that temperature dependence of scattering intensity is different between two scattering vectors. It may reflects a anisotropic electronic structure which originates from the orbital degrees of freedom in manganese oxides.
, which shows a metal-insulator transition, we found that temperature dependence of scattering intensity is different between two scattering vectors. It may reflects a anisotropic electronic structure which originates from the orbital degrees of freedom in manganese oxides.
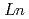
 Ca
Ca MnO
MnO (
(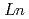 =Ho, Er, Tm, Yb and Lu)
=Ho, Er, Tm, Yb and Lu)Yoshii, Kenji; Abe, Hideki*; Ikeda, Naoshi*
Journal of Solid State Chemistry, 178(12), p.3615 - 3623, 2005/12
Times Cited Count:25 Percentile:67.33(Chemistry, Inorganic & Nuclear)no abstracts in English
 Sr
Sr MnO
MnO (
(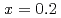 ,
, 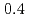 )
)Ishii, Kenji; Inami, Toshiya; Owada, Kenji; Kuzushita, Kaori; Mizuki, Junichiro; Murakami, Yoichi; Ishihara, Sumio*; Endo, Yasuo*; Maekawa, Sadamichi*; Hirota, Kazuma*; et al.
Physical Review B, 70(22), p.224437_1 - 224437_6, 2004/12
Times Cited Count:19 Percentile:64.32(Materials Science, Multidisciplinary)Electronic excitations in the hole doped manganese oxides (La Sr
Sr MnO
MnO ,
, 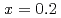 and
and 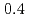 ) have been elucidated by using the resonant inelastic X-ray scattering (RIXS) method. A doping effect in the strongly correlated electron systems has been observed for the first time. The scattering spectra show that a salient peak appears in low energies indicating the persistence of the Mott gap. At the same time, the energy gap is partly filled by doping holes and the spectral weight energy shifts toward lower energies. The excitation spectra show little change in the momentum space as is in undoped LaMnO
) have been elucidated by using the resonant inelastic X-ray scattering (RIXS) method. A doping effect in the strongly correlated electron systems has been observed for the first time. The scattering spectra show that a salient peak appears in low energies indicating the persistence of the Mott gap. At the same time, the energy gap is partly filled by doping holes and the spectral weight energy shifts toward lower energies. The excitation spectra show little change in the momentum space as is in undoped LaMnO . On the other hand, the scattering intensities in the low energy excitations of
. On the other hand, the scattering intensities in the low energy excitations of 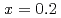 are anisotropic in temperature dependence, which indicates an anisotropy of magnetic interaction and underlying effect of the orbital.
are anisotropic in temperature dependence, which indicates an anisotropy of magnetic interaction and underlying effect of the orbital.
Hotta, Takashi; Moraghebi, M.*; Feiguin, A.*; Moreo, A.*; Yunoki, Seiji*; Dagotto, E.*
Physical Review Letters, 90(24), p.247203_1 - 247203_4, 2003/06
Times Cited Count:83 Percentile:91.22(Physics, Multidisciplinary)Novel ground-state spin structures in undoped and doped manganites are here investigated based on the orbital-degenerate double-exchange model, by using mean-field and numerical techniques. In undoped manganites, a new antiferromagnetic (AFM) state, called the E-type phase, is found adjacent in parameter space to the A-type AFM phase. Its structure is in agreement with recent experimental results. This insulating E-AFM state is competing with a ferromagnetic metallic phase as well, suggesting that large magneto-resistant effects could exist even in undoped manganese oxides. For doped layered manganites, the phase diagram includes another new AFM phase of the 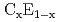 -type. Experimental signatures of the new phases are discussed.
-type. Experimental signatures of the new phases are discussed.
 (Ln=Ho, Er, Tm, Yb and Lu)
(Ln=Ho, Er, Tm, Yb and Lu)Yoshii, Kenji; Abe, Hideki*
Journal of Solid State Chemistry, 165(1), p.131 - 135, 2002/04
Times Cited Count:46 Percentile:84.59(Chemistry, Inorganic & Nuclear)no abstracts in English
 Ca
Ca MnO
MnO ; Evidence of two-phase coexistence
; Evidence of two-phase coexistenceYamada, Yasusada*; Iwase, Takeshi*; Wakahiki, Masaya*; Suzuki, Junichi
Journal of the Physical Society of Japan, 70(6), p.1593 - 1597, 2001/06
Times Cited Count:2 Percentile:20.74(Physics, Multidisciplinary)no abstracts in English
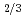 Ca
Ca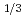 MnO
MnO
Wakahiki, Masaya*; Metoki, Naoto; Suzuki, Junichi; Oikawa, Kenichi; Nie, J.*; Tachiki, Masashi*; Yamada, Yasusada
Journal of the Physical Society of Japan, 70(4), p.1090 - 1098, 2001/04
Times Cited Count:6 Percentile:42.11(Physics, Multidisciplinary)no abstracts in English
Fernandez-Baca, J. A.*; Dai, P.*; Wakabayashi, Nobuyoshi*; Plummer, E. W.*; Katano, Susumu; Tomioka, Yasuhide*; Tokura, Yoshinori*
Journal of the Physical Society of Japan, Vol.70, Supplement A, p.85 - 87, 2001/00
no abstracts in English
Onuki, Toshihiko; Kozai, Naofumi
Radiochimica Acta, 68, p.203 - 207, 1995/00
no abstracts in English
 on manganese oxide formed by reduction of MnO
on manganese oxide formed by reduction of MnO

Kato, Tomoaki; Onuki, Toshihiko; Saito, Takumi*; Yu, Q.
no journal, ,
To develop a technology for recovering radioactive Co, we investigated the formation mechanism of Mn(IV) oxide (biomasss-MnO ) prepared using bacterial cells as reducing agent and sorption behavior of Co
) prepared using bacterial cells as reducing agent and sorption behavior of Co on biomasss-MnO
on biomasss-MnO . We found that biomasss-MnO
. We found that biomasss-MnO sorbs more Co than the MnO
sorbs more Co than the MnO prepared using lactic acid as reducing agent and the Co sorbed on biomass-MnO
prepared using lactic acid as reducing agent and the Co sorbed on biomass-MnO was oxidized to Co(III).
was oxidized to Co(III).
 into Mn oxides produced by MnO
into Mn oxides produced by MnO
 reduction using biomass
reduction using biomassKato, Tomoaki; Onuki, Toshihiko; Yu, Q.
no journal, ,
Various radionuclides were released into seawater by the accident of the Fukushima Daiichi Nuclear Power Plant. We need to establish techniques to eliminate such radionuclides from the contaminated seawater. Mn oxides are known to sorb various metal ions. Mn oxides can be produced by reduction of MnO
 using biomass (biomass-MnOX) although there is little information on metal uptake mechanisms during the formation of biomass-MnOX. In this research, we investigated the uptake of Sr
using biomass (biomass-MnOX) although there is little information on metal uptake mechanisms during the formation of biomass-MnOX. In this research, we investigated the uptake of Sr during the formation of biomass-MnOX produced by reduction of KMnO
during the formation of biomass-MnOX produced by reduction of KMnO with cells of Pseudomonas fluorescens. After inoculating the cells into the Mn(VII) and Sr
with cells of Pseudomonas fluorescens. After inoculating the cells into the Mn(VII) and Sr containing solution, blackish precipitates were formed in the bottom. EXAFS analysis of Sr
containing solution, blackish precipitates were formed in the bottom. EXAFS analysis of Sr in the precipitates showed that the presence of Mn around Sr, indicating that Sr was formed inner-sphere complex with Mn during coprecipitation.
in the precipitates showed that the presence of Mn around Sr, indicating that Sr was formed inner-sphere complex with Mn during coprecipitation.
Tanaka, Kazuya; Tani, Yukinori*; Onuki, Toshihiko*
no journal, ,
We investigated sorption of rare earth elements (REEs) and actinides on biogenic Mn oxide. Sorption experiments of REEs showed a negative Ce anomaly under circumneutral conditions. SEC-HPLC-ICP-MS analysis confirmed that Ce was complexed with organic ligands in solution. A line of data indicates that the negative Ce anomaly under circumneutral pH conditions arose from Ce(III) oxidation on biogenic Mn oxide and subsequent complexation of Ce(IV) with biomolecules. Th(IV) was sorbed, and then desorbed from biogenic Mn oxide with time. The desorption of Th(IV) is possibly attributed to organic ligands released from fungal cells as observed in the Ce sorption. In contrast, sorption of Pu(IV) increased with time, which was similar to that of U(VI). Plutonium(IV) was possible to be oxidized to Pu(VI) by Mn oxide. Sterilized biogenic Mn oxide did not show the desorption behavior of Th(IV), indicating that living cells would release organic ligands strongly complexed with Th(IV).
Takeda, Ayaka*; Nakano, Yuriko*; Ochiai, Asumi*; Yokoo, Hiroki*; Ohara, Yoshiyuki; Fukuyama, Kenjin; Nagayasu, Takaaki; Onuki, Toshihiko*; Utsunomiya, Satoshi*
no journal, ,
no abstracts in English
Tanaka, Kazuya; Kozai, Naofumi; Yamaji, Keiko*; Masuya, Hayato*; Grambow, B.
no journal, ,
Radium-226, a descendant nuclide of  U, is of serious concern in Ningyo-toge Environmental Engineering Center because its radioactive concentration in mining wastewater exceeds the effluent standard. In this study, we examined Ba and Sr adsorption on biogenic Mn oxide as a preliminary surrogate for Ra adsorption. Both Ba and Sr show the dependence of adsorption on NaCl concentration. Overall, Ba gave an order of magnitude higher Kd values than Sr. Adsorption behavior of Ra would be similar to that of Ba in terms of ionic radius. Barium showed Kd value greater than 10
U, is of serious concern in Ningyo-toge Environmental Engineering Center because its radioactive concentration in mining wastewater exceeds the effluent standard. In this study, we examined Ba and Sr adsorption on biogenic Mn oxide as a preliminary surrogate for Ra adsorption. Both Ba and Sr show the dependence of adsorption on NaCl concentration. Overall, Ba gave an order of magnitude higher Kd values than Sr. Adsorption behavior of Ra would be similar to that of Ba in terms of ionic radius. Barium showed Kd value greater than 10 in 10 mM NaCl solution, similar to the ionic strength of the fresh water system in the Ningyo-toge center. Therefore, it is expected that the biogenic Mn can work effectively for removal of Ra from mining wastewater.
in 10 mM NaCl solution, similar to the ionic strength of the fresh water system in the Ningyo-toge center. Therefore, it is expected that the biogenic Mn can work effectively for removal of Ra from mining wastewater.